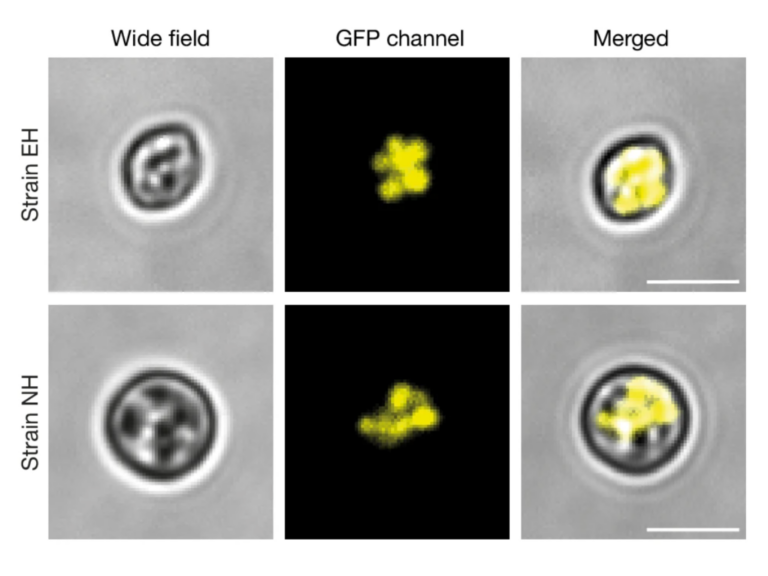
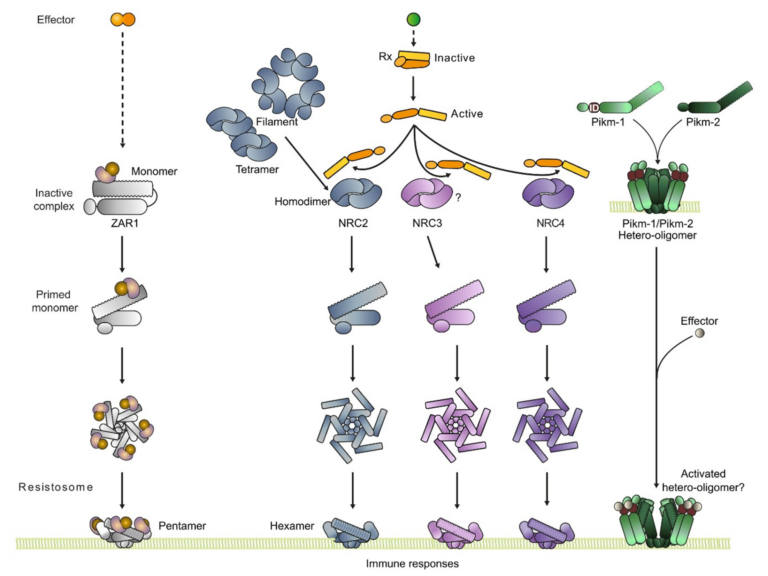
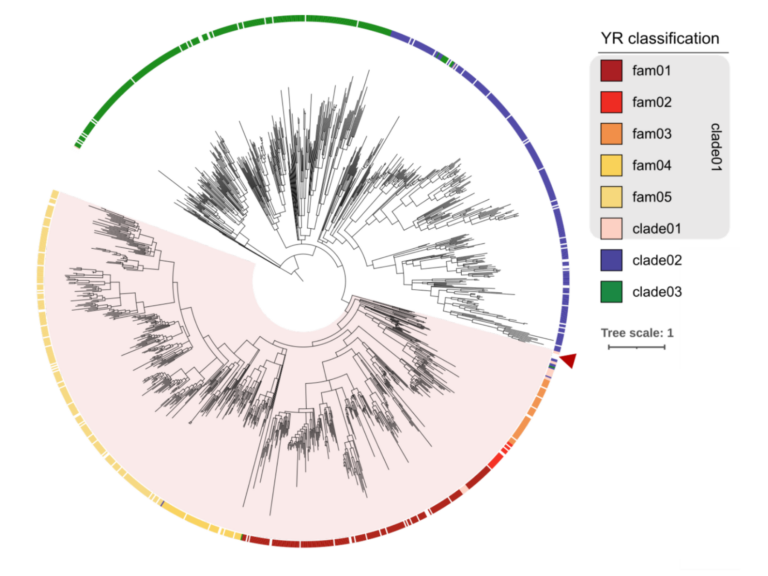
The N-terminal executioner domains of NLR immune receptors are functionally conserved across major plant lineages
Nucleotide-binding domain and leucine-rich repeat (NLR) proteins are a prominent class of intracellular immune receptors that are present across diverse plant lineages. However, our understanding of plant NLR structure and function is limited to the evolutionarily young flowering plant clade (angiosperms). Here, we describe an extended spectrum of NLR diversity across major plant lineages and demonstrate functional conservation of N-terminal ‘executioner’ domains that trigger immune responses. We show that broadly distributed CC (coiled-coil) and TIR (toll/interleukin-1 receptor) domains retain executioner function through trans-lineage activation of immune-related cell death in the model angiosperm Nicotiana benthamiana. Further examination of a CC subfamily specific to non-flowering lineages uncovered an essential N-terminal MAEPL motif with functional similarity to resistosome-forming CC-NLRs. Ectopic activation of the MAEPL-type CC in the divergent liverwort Marchantia polymorpha led to profound growth inhibition, defense gene activation, and signatures of cell death resembling CC activity in flowering plants. Moreover, comparative macroevolutionary transcriptomics in Marchantia and Nicotiana identified conserved CC responsive genes, providing further insight into the core aspects of CC function shared between flowering and non-flowering plants. Our findings highlight the need to understand NLR structure and function across the full spectrum of plant diversity.